Inorganic Thickeners in Cosmetics
- parag kale
- May 7, 2021
- 2 min read
Updated: May 23, 2021
Many formulators use inorganic thickeners in the cosmetic products. But they are not used just for the thickening property, some of them have so many other benefits:
Many of them are used to obtain thixotropy in a system.
Some thickeners are used to suspend the solid particles.
Used for stabilization of creams and suspensions.
Most can be used as binding agent in pilling operations.
Some inorganic thickeners used as parting or conditioning agents to prevent agglomeration and caking in powder products.
Many inorganic thickeners are used to absorb moisture and facial oil, also to minimize shine.
They are also use for improving feel of product.

On the basis of source of mineral can be classified as
A) Natural Clay minerals
These types of minerals are produced from certain natural clay minerals, attapulgite, sepiolite, montmorillonite, hectorite, and saponite. These clay mineral’s crystals composed of two basic structure units:
Hydrated alumina
Hydrated silica sheets
The hydrated alumina sheet consists of oxygen or hydroxyls in octahedral coordination around aluminum, iron, and/or magnesium. If there is only aluminum cation then called gibbsite sheet, and if magnesium is the only cat ion then is called brucite sheet.

Hydrated silica sheet has built its structural unit by silicon ion, equal distance from four oxygen in a tetrahedral configuration. These groups form a repeated network of tetrahedral bases which are in one plane.

These two basic sheets units are connected and form crystalline particles of the various clay minerals. Sheets form three layer mineral groups of two silica tetrahedral sheets with one central octahedral sheet. The bonds between silica-alumina-silica sheets are weak, water and other polar organic compounds can enter between the crystal layers, expanding the lattice.
Mechanism of Thickening
1. Hydration of clays with formation of surface layers of oriented water.
2. Hydration of cations in exchange positions.
3. Hydration of clays with expansion of lattices
4. Mechanical thickening.
5. Hydrogen bonding.
B) Organic modified clay minerals
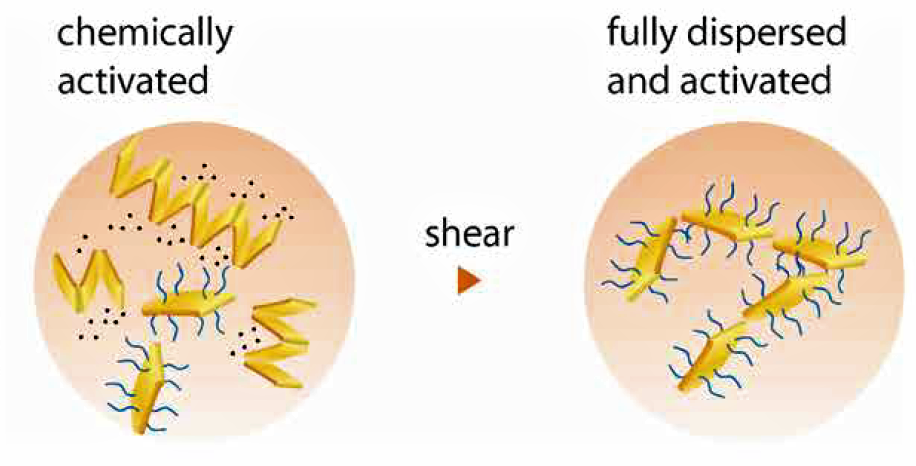
The natural clay minerals play an excellent role in increasing the viscosity of polar solvent but these are unable to thicken the nonpolar system. So, for making them hydrophobic thickening agent they need some modification onto their surface structure.
Organic molecules have the capacity to improve the dispersion in nonpolar liquids because of their unique amphiphilicity. They have a polar group on one end a non-polar group on the other, so the polar group is adsorbed to the hydrophilic surface of the clay and remaining lipophilic group will adsorb on the non-polar material.
Cosmetics market has many thickening agents available, which are based on bentonite, hectorite and attapulgite. They are organically modified during production by the manufacturer. Typical modifying compounds are quaternary amines, tertiary amines, amine acetates, imidazolines, fatty sulfates, amine oxides etc.
Modification can also be accomplished by the ultimate user by simple addition of certain surfactants during dispersion of the thickeners.









Comments